Caffeine and Camellia sinensis enhance cognition and decrease acetylcholinesterase activity in scopolamine-induced memory loss in female Swiss mice
DOI:
https://doi.org/10.23921/amp.2022v6i2.00062Keywords:
Acetylcholinesterase, Caffeine, Green tea, Memory loss, ScopolamineAbstract
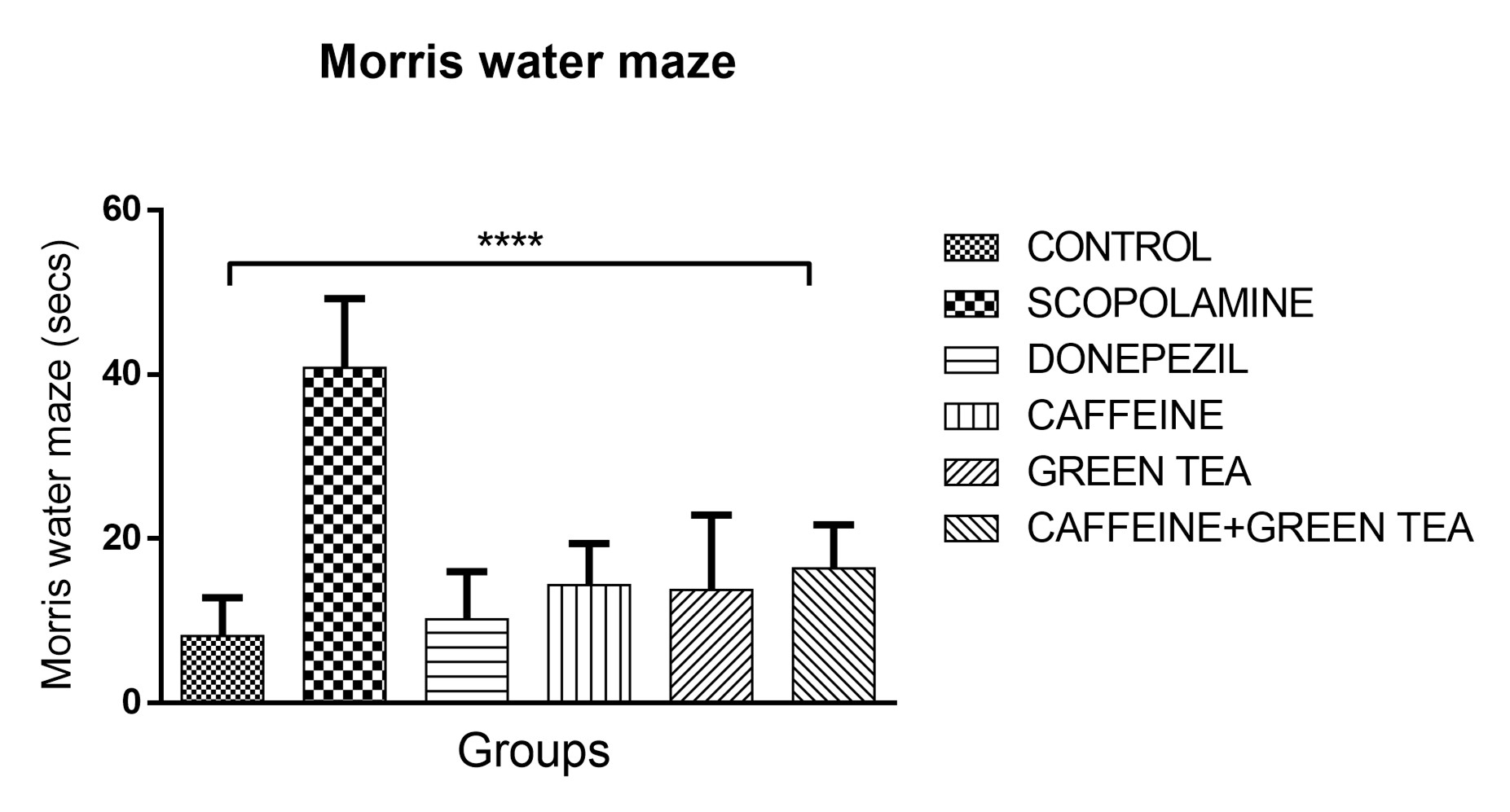
Caffeine and Camellia sinensis (green tea) has been known to have positive effect on memory. The present study investigated the possible effect of caffeine and green tea co-administration on oxidative stress markers, inflammatory marker and acetylcholine esterase activity in scopolamine-induced memory loss in female Swiss mice. Memory behavioral tests using Y-maze and Morris water maze was carried out, followed by oxidative stress biomarkers, acetylcholinesterase activity and Tumor Necrosis Factor alpha (TNF-?) evaluation from the mice brain tissues after caffeine and green tea administration. Scopolamine administered intraperitoneally at a dose of 1mg/kg Body Weight (BW) for 7 days significantly reduced the percent alternation of the mice in Y-maze thus, increased acetylcholinesterase activity and increased TNF-? level. However, caffeine administered orally at a dose 50mg/kg BW and green tea administered orally at a dose of 60mg/kg BW increased the percent alternation significantly, reduced acetylcholinesterase activity and reduced the TNF-? level significantly. Oxidative stress markers evaluated GSH and MDA, showed no significant difference across all groups. These findings showed scopolamine has a deteriorating effect on cognition by increasing acetylcholinesterase activities thus mopping out acetylcholine at a faster rate. However, caffeine and green tea singly and in combination restored cognition, reduced acetylcholinesterase activity and restored TNF-? level. The neuroprotective effect of caffeine and green tea was compared to that of Donepezil, an established cognition enhancing drug and the effect was agonistic. The ability of caffeine and green tea to reduce acetylcholinesterase activity could be the mechanism for the ability to enhance memory. The ability of these compounds in restoring TNF-? level also further potentiates their neuroprotective capability.
Downloads
References
Burgess N. Spatial cognition and the brain. Ann N Y Acad Sci. 2008;1124:77-97.
Walsh E, Oakley DA, Halligan PW, Mehta MA, Deeley Q. Brain mechanisms for loss of awareness of thought and movement. Soc Cogn Affect Neurosci. 2017;12(5):793-801.
Hamilton RJ. Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. 16th ed. Burlington: Tarascon Publishing (an imprint of Jones & Bartlett Learning). 2015; p. 270.
Falsafi SK, Deli A, Höger H, Pollak A, Lubec G. Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. PLoS One. 2012;7(2):e32082.
Schmeller T, Sporer F, Sauerwein M, Wink M. Binding of tropane alkaloids to nicotinic and muscarinic acetylcholine receptors. Pharmazie. 1995;50(7):493-495.
Pushpalatha B, Venumadhav N, Swathi M, Raju BA. Neuroprotective effect of resveratrol against scopolamine-induced cognitive impairment and oxidative stress in rats. Arch Biol Sci. 2013;65(4):1381-1386.
Barak Y, Savorai O, Mavashev S, Beni A. Animal-assisted therapy for elderly schizophrenic patients: a one-year controlled trial. Am J Geriatr Psychiatry. 2001;9(4):439-442.
Cacabelos R. Donepezil in Alzheimer's disease: From conventional trials to pharmacogenetics. Neuropsychiatr Dis Treat. 2007;3(3):303-333.
Alasmari F. Caffeine induces neurobehavioral effects through modulating neurotransmitters. Saudi Pharm J. 2020;28(4):445-451.
Cappelletti S, Piacentino D, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. 2015;13(1):71-88.
Dall'Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp Neurol. 2007;203(1):241-245.
Dall'Igna OP, Souza DO, Lara DR. Caffeine as a neuroprotective adenosine receptor antagonist. Ann Pharmacother. 2004;38(4):717-718.
Khan N, Mukhtar H. Tea and health: studies in humans. Curr Pharm Des. 2013;19(34):6141-6147.
Sutherland WJ, Armstrong-Brown S, Armsworth PR, Tom B, Brickland J, Campbell CD, Chamberlain DE, Cooke AI, Dulvy NK, Dusic NR, Fitton M, Freckleton RP, Godfray HC, Grout N, Harvey HJ, Hedley C, Hopkins JJ, Kift NB, Kirby J, Kunin WE, Macdonald DW, Marker B, Naura M, Neale AR, Oliver T, Osborn D, Pullin AS, Shardlow ME, Showler DA, Smith PL, Smithers RJ, Solandt J, Spencer J, Spray CJ, Thomas CD, Thompson J, Webb SE, Yalden DW, Watkinson AR. The identification of 100 ecological questions of high policy relevance in the UK. J Appl Ecol. 2006;43(4):617-627.
Yadang FS, Nguezeye Y, Kom CW, Betote PH, Mamat A, Tchokouaha LR, Taiwé GS, Agbor GA, Bum EN. Scopolamine-induced memory impairment in mice: Neuroprotective effects of Carissa edulis (Forssk.) Valh (Apocynaceae) aqueous extract. Int J Alzheimers Dis. 2020;2020:6372059.
Wu B, Wei Y, Wang Y, Su T, Zhou L, Liu Y, He R. Gavage of D-Ribose induces A?-like deposits, Tau hyperphosphorylation as well as memory loss and anxiety-like behavior in mice. Oncotarget. 2015 Oct 27;6(33):34128-34142.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70-77.
Onaolapo OJ, Onaolapo AY, Josiah T, Osaku M, Akanji OO, Abiodun OR. Elevated plus maze and Y-maze behavioral effects of subchronic, oral low dose monosodium glutamate in swiss albino mice. IOSR J Pharm Biol Sci. 2012;3(4):21-27.
Giovannini MG, Spignoli G, Carlà V, Pepeu G. A decrease in brain catecholamines prevents oxiracetam antagonism of the effects of scopolamine on memory and brain acetylcholine. Pharmacol Res. 1991;24(4):395-405.
Chapouthier G, Lepicard E, Rössler A, Venault P. Anxiety, a bridge between epilepsy and memory? Philos Sci. 2002;6(1):75-91. [Article in French]
Blake MG, Krawczyk MC, Baratti CM, Boccia MM. Neuropharmacology of memory consolidation and reconsolidation: Insights on central cholinergic mechanisms. J Physiol Paris. 2014;108(4-6):286-291.
Published
How to Cite
License
Copyright (c) 2022 Quench Academy of Medical Education and Research (QAMER)

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors warrants and represents that the submitted MANUSCRIPT is an original work and has not been published before in any form, and that it does not infringe upon any copyright or other right(s), that it does not contain infringing, libelous, obscene or other unlawful matter, that he/she is the sole and exclusive owner of the rights here-in conveyed to the Publisher, and that he/she has obtained the customary permission from the copyright owner or his legal representative whenever a text/passage from copyrighted material is quoted or a table or illustration from such material is used. The Author(s) will indemnify the Publisher for, and hold the Publisher harmless from any loss, expense or damage occasioned by any claim or suit by a third party for copyright infringement or arising out of any breach of the foregoing warranties as a result of publication of the Article. The Article shall be delivered to the Publisher free of copyright charges. In the event that the Article is not accepted and published by Publisher, this agreement becomes null and void.
Sherpa/Romeo publisher policy can be viewed at Annals of Medical Physiology - Sherpa/Romeo Policy
Plum X metrics
Article level metrics are shown here









