Immunoglobulin stimulatory property of aqueous leaf extract of Justicia carnea in male Wistar rats exposed to lead acetate
Immunoglobulin stimulatory-property of Justicia carnea extract in male Wistar rats
Revised : 2023-09-07
Accepted : 2023-10-01
Online : 2023-10-23
Print : 2023-10-24

Full text
Abstract
With regards to previously speculated hematinic/hematopoietic potentials of Justicia carnea, the present study set out to evaluate the effect of the administration of the aqueous leaf extract of Justicia carnea on the immunoglobulins levels in two sets of rats (non-lead intoxicated and lead- intoxicated rats). Forty (40) male Wistar rats were obtained and randomly distributed into eight (8) groups of 5 rats each as follows: Group 1 served as negative control/received 1ml of distilled water daily; Group 2 served as positive control/treated with 10mg/kg.bw of lead acetate (Pb); Groups 3 to 5 received 400, 600 and 800mg/kg body weight of aqueous leaf extract of Justicia carnea respectively daily and Groups 6 to 8 received 400, 600 and 800mg/kg bw extract + 10mg/kg.bw Pb respectively daily. At the end of 6 weeks of treatment, blood samples were harvested from the study animals via cardiac puncture after sedating them with 80% chloroform for laboratory analyses. The result of the present study indicated that immunoglobulins (Ig) G, M and A had significant (p<0.05) increases across all extract treated rats. In conclusion, this study has shown that extract possess humoral immunity enhancing potential. However, extrapolating from the present study, the frequency/consumption of the high dose of the extract should be checked in its ethnobotanical application as to prevent any possible consequent undesirable effects of autoimmune conditions.
Keywords: Humoral immunity, Immunoglobulins, Justicia carnea, Toxicity
How to cite: Ojeka SO, Onyekwere JU, Obia O, Bekinbo MT. Immunoglobulin stimulatory property of aqueous leaf extract of Justicia carnea in male Wistar rats exposed to lead acetate. Ann Med Physiol. 2023;7(1):1-7. doi: 10.23921/amp.2023v7i1.00070
Introduction
It has been submitted that hematological disorders are common in low-income countries and that they have a considerable impact on the morbidity and mortality of individuals in such populations with the attendant negative effect on the growth and development of their countries [1,2,3]. In addition, the high rates of illiteracy, scarcity of adequate orthodox medications and the fear of their side effects on chronic usage, a large chunk of the population of low-income countries have continued to rely on herbal therapies [4,5,6].
Thus, in line with the foregoing, the leaf extract of Justicia carnea (J. carnea) has been severally reported not only to be edible, safe and rich in beneficial nutrients with no traceable evidence of biologic toxicities but also possesses antianemic potentials [7,8]. Justicia carnea belongs to the family, Acanthanceae. The genus Justicia is of Scotish origin. It is a flowering plant that is widely cultivated in Tropical Africa and in Nigeria, Justicia carnea, has been identified as a medicinal plant that serve as a quick blood tonic and other related disorders [9,10,11].
More so, the ethnobotanical application of J. carnea in the treatment or management of several other ailments and disease conditions, including anemia, dyslipidemia, amongst others, is well documented in Nigeria [7,12]. The general indication of these earlier reports is that the J. carnea plant may be of high therapeutic value in the treatment of hematological, lipid and oxidative stress related abnormalities with perhaps no adverse effects on the endogenous/biological system [13,14]. Virtually all of the aforementioned therapeutic potentials of J. carnea are attributed to the abundant combination of rich phytochemicals such as saponin, flavonoids, alkaloids, phenols, steroids amongst others [15,16,17,18]. Conversely, as emphatic and beneficial as the foregoing preliminary reports on J. carnea on hematological related disorders, not much attention has been given to its possible effects on the basic aspects of immune physiology in mammalian models.
Again, a close link is said to exist between immune physiology complexities and infections which are a major cause of illness and death in our population [3]. More so, considering the fact that, the reported rich phytochemical constituents of the J. carnea plant [12] and their respective effects (like phenolics, etc.,) possess anti-cancer, anti-inflammatory, anti-allergic and estrogenic effects [15], the present study set out to evaluate the effect of the administration of the leaf extract of Justicia carnea on immunoglobulins (Ig) A, E, M and G (which constitute a basic aspect of immune physiology) levels in male Wistar rats exposed to lead acetate.
Materials and methods
Research design
The present study was experimental/laboratory-based and used only animal model, male Wistar rats, obtained and housed in the Animal House of the Faculty of Basic Medical Sciences, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria. The animals weighed between 160g and 200g and were maintained in the 12-hour light/dark cycle under room temperature and about ambient humidity. They were acclimatized to the new setup for 14 days before the commencement of experimentations; and were also allowed access to rat feed and tap water ad libitum. The guideline for the care and use of experimental animals was strictly followed [19]. Ethical approval for the study was obtained on the 20th of December, 2022 from the Central Ethics Committee for Research Management and Development, University of Port Harcourt, Nigeria with reference number: UPH/CEREMAD/REC/MM86/022.
Plant materials
The leaves of Justicia carnea were collected from a private garden located in Port Harcourt, Rivers State, Nigeria. A specimen of the Justicia carnea leaves were authenticated at the herbarium section of the Department of Plant Science and Biotechnology, University of Port–Harcourt and herbarium number, UPH/C/096, issued.
Aqueous extraction of Justicia carnea leaves
The aqueous extraction of the Justicia carnea leaves was preferred for this study over ethanol or methanol extraction due to the fact that the most widely and frequently ethnobotanical applications of the plant is via aqueous extraction chosen [8]. Justicia carnea leaves were air dried under shade to prevent the ultraviolet decomposition of volatile and light sensitive photochemical constituents of the plant. After the leaves got well dried, they were pulverized into fine powder. The obtained dried powder of the plant was then extracted using the cold maceration method of Sankeshwari [20]. Two hundred grammes (200g) of the blended powder were dissolved in 1liter of water in a 2L conical flask. After 72 hours of periodic macerations, the mixture was filtered using the whatman number 1 filter (Whatman England) and cotton wool to isolate plant sample parcels. The rotary evaporator and water bath at 40°C respectively were used to drive off the remaining water and the recovered semi-solid extract was then kept in sterile screw-capped container, properly labeled and refrigerated at 4°C.
Dosage preparation of test samples
The dosages of 400, 600 and 800mg/kg b.w were adopted by the present study based on the report of Anthonia et al [8], who reported the LD50 of the Justicia carnea leaves to be above 5000mg/kg. It is important to state here that the doses of 600mg/kg and 800mg/kg of the extract and 10mg/kg of the lead acetate (Pb(C2H3O2)2) were determined using standard mathematical computations. Again, the administration of 10mg/kg of lead acetate (Pb(C2H3O2)2) in the study models via their drinking water commenced for two weeks prior to the commencement of the administration of the extracts and continued through the entire period of administration.
Study protocol
Following the acclimatization of the 40 male Wistar rats, they were randomly distributed into eight (8) groups of five (5) rats each and were labeled as follows:
- Group 1: served as negative control and received only 1ml of distilled water daily.
- Group 2: served as positive control and were treated with lead acetate (Pb) + 1ml of distilled water daily for 6 weeks.
- Group 3: received 400mg/kg body weight (b.w) of aqueous leaf extract of Justicia carnea (ALEJC) for 6 weeks.
- Group 4: received 600mg/kg b.w ALEJC for 6 weeks.
- Group 5: received 800mg/kg b.w ALEJC for 6 weeks.
- Group 6: received 400mg/kg b.w ALEJC + Pb for 6 weeks.
- Group 7: received 600mg/kg b.w ALEJC + Pb for 6 weeks.
- Group 8: received 800mg/kg b.w ALEJC + Pb for 6 weeks.
Harvesting of samples from study animals
At the end of the treatment duration, using the method of cardiac puncture, the study animals were harvested of blood samples for laboratory analyses after sedating them with 80% chloroform soaked in a cotton wool inside a desiccator. About 5ml of blood was taken per rat and put into properly labeled appropriate sample bottles before the automated analyses of the immunoglobulins levels using the method used by Amah-Tariah et al [21].
Method of data analysis
The data obtained from the present study were subjected to statistical analyses using the Statistical Package for Social Sciences (SPSS) version 21.0. Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Post-Hoc multiple comparison test and p< 0.05 was considered statistically significant. The values were expressed as mean ± standard error of mean (SEM).
Results
Figure 1 shows the result on the effect of administration of aqueous leaf extract of Justicia carnea (ALEJC) on immunoglobulin (Ig) G levels in male Wistar rats. The changes in the IgG levels, apart from groups 3 and 6 with no significant (p>0.05) difference, all other test groups showed significant (p<0.05) elevations in their IgG levels compared to that of group 1. These increases were highest in groups 4 and 2 respectively. Figure 2 shows the result on the effect of administration of aqueous leaf extract of Justicia carnea (ALEJC) on IgM levels in male Wistar rats. The result indicated marked (P<0.05) elevation in IgM levels of groups 6, 7 and 8 had significant (p<0.05) elevations compared to groups 1 and 2 (although the elevation in group 6 was not significant compared to that of group 2). These increases were greatest in groups 7 and 8. Groups 4 and 5 had remarkable (p<0.05) reductions with respect to group 2. On the other hand, groups 6, 7 and 8 had elevated IgM values when compared to groups 4 and 5.
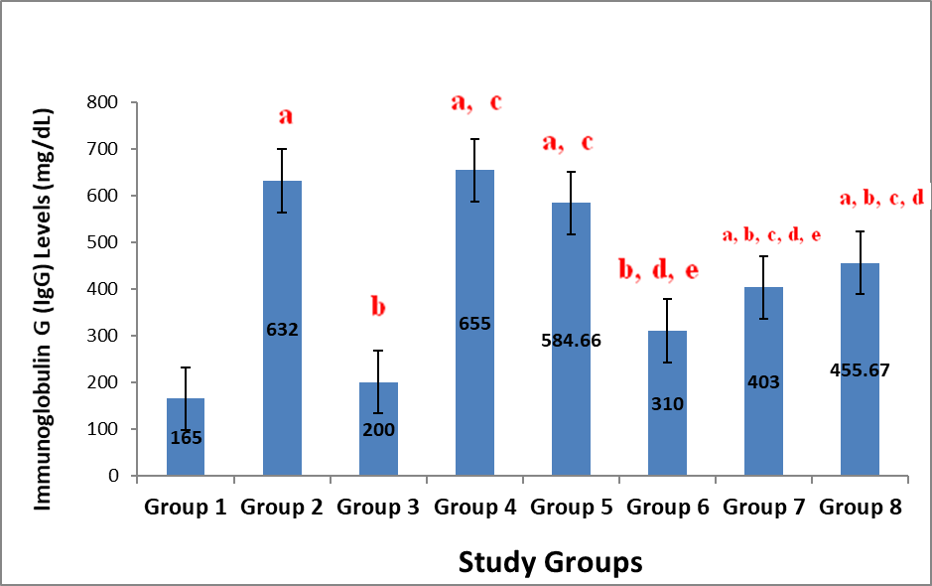
Note: Values represent mean ± SEM, n=6; a=Significant at p<0.05 compared to Group 1; b=Significant at p<0.05 when compared to group 2; c=Significant at p<0.05 when compared to group 3; d=Significant at p<0.05 when compared to group 4; e=Significant at p<0.05 when compared to group 5; f=Significant at p<0.05 when compared to group 6; g=Significant at p<0.05 when compared to group 7.)
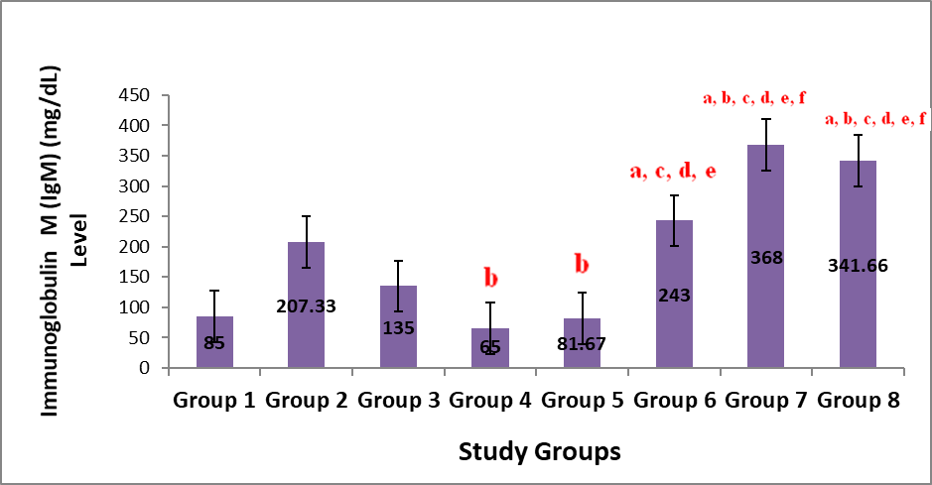
Note: Values represent mean ± SEM, n=6; a=Significant at p<0.05 compared to Group 1; b=Significant at p<0.05 when compared to group 2; c=Significant at p<0.05 when compared to group 3; d=Significant at p<0.05 when compared to group 4; e=Significant at p<0.05 when compared to group 5; f=Significant at p<0.05 when compared to group 6; g=Significant at p<0.05 when compared to group 7.)
Figure 3 shows the result on the effect of administration of aqueous leaf extract of Justicia carnea (ALEJC) on IgA levels in male Wistar rats. On the changes in IgA levels, groups 2, 4, 5, 7 and 8 showed marked (p<0.05) elevations when compared to group 1. And only groups 5, 7 and 8 indicated significant increases in the IgA levels compared to that of group 3.
Figure 4 shows the result on the effect of administration of aqueous leaf extract of Justicia carnea (ALEJC) on IgE levels in male Wistar rats. The IgE level varied non-significantly (P>0.05) in a non-uniform manner across the various doses except that of group 4 that was significantly (P<0.05) reduced when compared to all other groups. Similarly, group 8 was markedly reduced (P<0.05) when compared to that of 3.The IgE level varied non-significantly (P>0.05) in a non-uniform manner across the various doses except that of group 4 that was significantly (P<0.05) reduced when compared to all other groups. Similarly, group 8 was markedly reduced (P<0.05) when compared to that of 3.
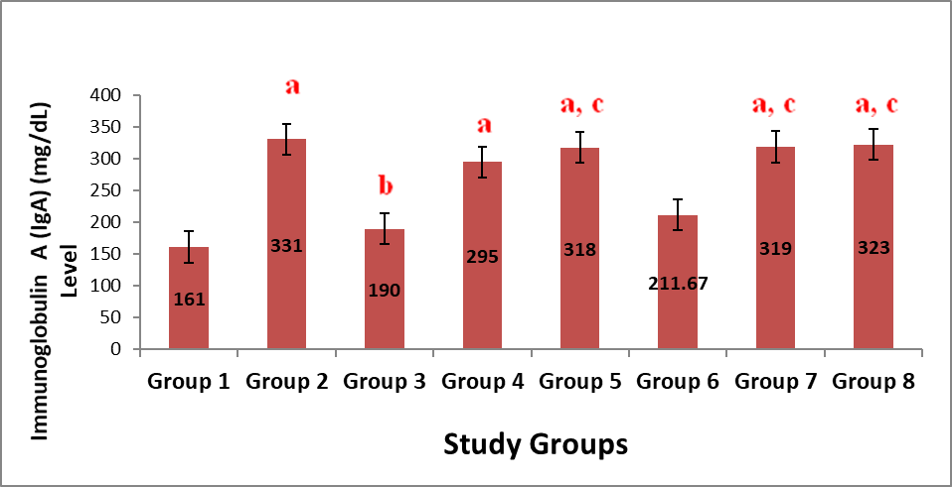
Note: Values represent mean ± SEM, n=6; a=Significant at p<0.05 compared to Group 1; b=Significant at p<0.05 when compared to group 2; c=Significant at p<0.05 when compared to group 3; d=Significant at p<0.05 when compared to group 4; e=Significant at p<0.05 when compared to group 5; f=Significant at p<0.05 when compared to group 6; g=Significant at p<0.05 when compared to group 7.)
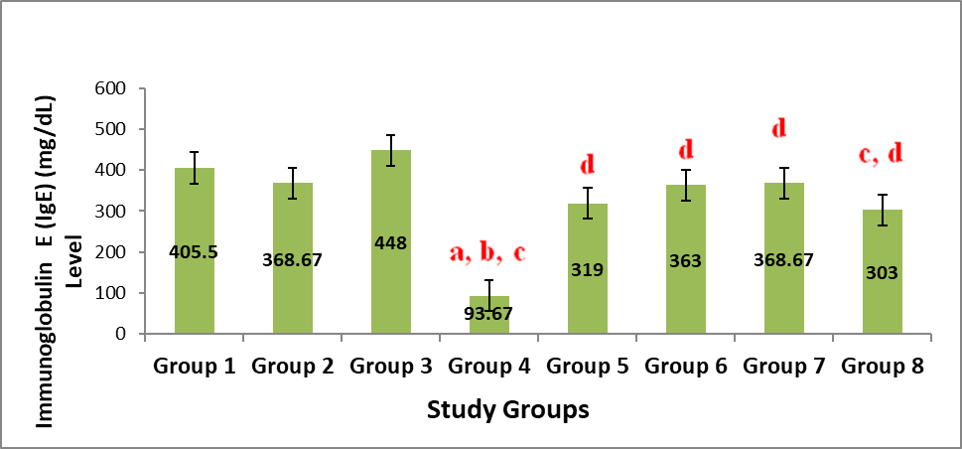
Note: Values represent mean ± SEM, n=6; a=Significant at p<0.05 compared to Group 1; b=Significant at p<0.05 when compared to group 2; c=Significant at p<0.05 when compared to group 3; d=Significant at p<0.05 when compared to group 4; e=Significant at p<0.05 when compared to group 5; f=Significant at p<0.05 when compared to group 6; g=Significant at p<0.05 when compared to group 7.)
Discussion
Antibodies, also known as immunoglobulins, are crucial for defending against infectious agents; various disease conditions manifest when these glycoproteins are lacking [22]. Immunoglobulins are released into the tissue space where they bind and neutralize their target antigens [23]. They are also known to be able to kill or destroy microbes/foreign invaders using 3 possible mechanisms namely neutralization, opsonization, and complement activation [24]. Considering their highly complex and specific roles, the search for natural agents that beneficially regulate them remain very important. Thus, the present study has made some useful explorations using J. carnea plant on a mammalian model, Wistar rats, and discussed them in the following paragraphs.
The result of the present study on the effect of administration of aqueous leaf extract of J. carnea (ALEJC) on immunoglobulin (Ig) levels in male Wistar rats, revealed significant IgG, IgM and IgA elevations in both lead-intoxicated and 600 and 800mg/kg ALEJC treated animals. The 600mg/kg ALEJC treated animals (group 4) were observed to have markedly reduced level of IgE; this decrease was dissimilar with IgE levels of groups 3 and 5 (treated with 400 and 800mg/kg ALEJC) thus making it surprising to note. Deductively, the use of higher doses of ALEJC in mammalian models should be checked considering this tendency to reduce IgE levels in the study animals.
It is known that changes in the levels of immunoglobulins are mainly influenced by the absence or presence of invading pathogens or foreign particles and by certain medications [25,26,27]. The effect of increasing doses of ALEJC on IgG, IgM (specifically in the ALEJC and Pb co-treated groups), IgA appears to be directly proportional. This finding is in line with an earlier submission by Yamada et al, [28] which stated that, by influencing different immune system components, herbs can strengthen the body and boost resistance to infections whether used as food or medicine. Thus, the ALEJC investigated by the current study may be renowned for its possible capacity to improve immunological performance, perhaps through stimulating innate immune responses. The finding of the present study on the immunoglobulins can be said to be unique in the sense that similar findings from previous studies are scarce or non-existent. With such a possibility, it will also be necessary to suggest that, a finger print identification of the actual active ingredient(s) possibly responsible for this effect on the IgG, IgM and IgA levels be carried out in further study (ies). This will, of course, be useful in the standardization of the possible therapeutic agent from the ALEJC. Consequently, it may be important to state that ALEJC may possess a stimulatory effect on IgG, IgM and IgA levels. Again, this finding has also shown the need for caution in the consumption of increasing doses of the ALEJC in mammalian models in order to prevent unbeneficial immunostimulations and its associated complexities. This is because, being an integral portion of the adaptive immune system [29,30,31], unchecked elevated levels of these immunoglobulins may over time result in autoimmune disorders. Meanwhile, the observed properties of the ALEJC may be helpful to immunodepressed conditions.
Conclusion
Considering the elevations in the levels of IgG, IgM and IgA in both lead-exposed and non-lead-exposed rats at higher doses of ALEJC; it is indeed suggested that the ALEJC possess the potential to elicit immunostimulatory effect on the humoral immunity. This property of the ALEJC truly call for further investigation of the actual active ingredients of the plant as to understand if they could become possible immunostimulatory candidates derived from natural source.
References
- Safiri S, Kolahi AA, Noori M, Nejadghaderi SA, Karamzad N, Bragazzi NL, Sullman MJM, Abdollahi M, Collins GS, Kaufman JS, Grieger JA. Burden of anemia and its underlying causes in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. J Hematol Oncol. 2021 Nov 4;14(1):185. [Crossref] [Pubmed] [Pubmed Central]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020 Oct 17;396(10258):1204-1222. [Crossref] [Pubmed] [Pubmed Central] Erratum in: Lancet. 2020 Nov 14;396(10262):1562.
- Thachil J, Owusu-Ofori S, Bates I. Haematological Diseases in the Tropics. In: Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White NJ, editors. Manson’s Tropical Infectious Diseases. Chapter 65, 2014;894-932.e7. [Crossref] [Pubmed Central] Epub 2013 Oct 21.
- James PB, Wardle J, Steel A, Adams J. Traditional, complementary and alternative medicine use in Sub-Saharan Africa: a systematic review. BMJ Glob Health. 2018 Oct 31;3(5):e000895. [Crossref] [Pubmed] [Pubmed Central]
- Njume C, Goduka NI. Treatment of diarrhoea in rural African communities: an overview of measures to maximise the medicinal potentials of indigenous plants. Int J Environ Res Public Health. 2012 Oct 26;9(11):3911-33. [Crossref] [Pubmed] [Pubmed Central]
- Okaiyeto K, Oguntibeju OO. African herbal medicines: Adverse effects and cytotoxic potentials with different therapeutic applications. Int J Environ Res Public Health. 2021 Jun 2;18(11):5988. [Crossref] [Pubmed] [Pubmed Central]
- Onyeabo C, Achi NK, Ekeleme-Egedigwe CA, Ebere CU, Okoro CK. Haematological and biochemical studies on Justicia carnea leaves extract in phenylhydrazine induced-anemia in albino rats. Acta Sci Pol Technol Aliment. 2017 Apr-Jun;16(2):217-230. [Crossref] [Pubmed]
- Anthonia OC, Ikechukwu UR, Uzoma NO, Uchenna Sunday EL. Nutritive properties of aqueous extract Justicia carnea leaves and its effects on haematological and some biochemical indices of anaemia induced male wistar albino rats. Biomedical Research. 2019;30(4). doi:10.35841/biomedicalresearch.30-18-666.
- Faluyi O. Nigeria’s medicinal plant: Justicia carne (Ewe eje). Punch 2021 Jun 13. Available from: https://punchng.com/nigerias-medicinal-plant-justicia-carne-ewe-eje/ (Accessed online in January, 2023)
- Minaopunyea O-OB, Chimeziea OH. Ameliorative potentials of Justicia carnea and Cnidoscolus aconitifolius on the fecundity of chloramphenicol-induced lymphoma rats. Journal La Lifesci. 2022 Mar 30;3(2):65-75. [Crossref]
- Imohiosen O. Phytochemical analysis on aqueous leaf extract of Justicia carnea (Acanthaceae) and its antibacterial activity on some isolated bacterial. American Journal of Food Science and Technology. 2023 May 7;2(1):16-20. [Crossref]
- Akintimehin ES, Karigidi KO, Omogunwa TS, Adetuyi FO. Safety assessment of oral administration of ethanol extract of Justicia carnea leaf in healthy wistar rats: hematology, antioxidative and histology studies. Clinical Phytoscience. 2021 Jan 3;7(1). [Crossref]
- Ani ON, Udedi SC, Asogwa KK, Enemali MO, Onwelumadu CM, Ikedife KS. Inhibitory potential and antidiabetic activity of leaf extracts of Justicia carnea. International Journal of Biochemistry Research & Review. 2020 Jun 30;34-45. [Crossref]
- Ukpabi-Ugo JC, Ndukwe PA, Iwuoha AG. Hepatoprotective effect of methanol extract of Justicia carnea leaves on carbon tetrachloride-intoxicated albino rats. Biochemistry & Analytical Biochemistry. 2019;8(2):381. [Crossref]
- Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chemistry. 2007;104(4):1372-8. [Crossref]
- Wood J, Yasmin-Karim S, Moreau M, Kumar R, Akwanwi J, Derek A, Atoneche F, Kress J, Ngwa AW. Characterization of isolated extracts from Justicia plant leaves used as remedy for anemia. Molecules. 2020 Jan 25;25(3):534. [Crossref] [Pubmed] [Pubmed Central]
- Ajuru MG, Kpekot KA, Robinson GE, Amutadi MC. Proximate and phytochemical analysis of the leaves of Justicia carnea Lindi and Justicia secunda Vahl and its taxonomic implications. Journal of Biomedicine and Biosensors. 2022 Dec 15;2(1):1-1.
- Okocha BI, Orie KJ, Duru RU, Ngochindo RL. Analysis of the active metabolites of ethanol and ethyl acetate extract of Justicia carnea. African Journal of Biomedical Research. 2023 Jul 14;26(1):109-17.
- National Research Council [NRC]. Guidelines of the National Institute of Health (NIH) for care and use of laboratory/research animals. 2011. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54050. (Accessed January, 2023)
- Sankeshwari RM, Ankola AV, Bhat K, Hullatti K. Soxhlet versus cold maceration: Which method gives better antimicrobial activity to licorice extract against Streptococcus mutans? Journal of the Scientific Society. 2018 May-Aug;45(2):67-71. [Crossref]
- Amah-Tariah FS, Bekinbo MT, Dapper DV. Comparative study of serum immunoglobulin levels in healthy pregnant and pregnant subjects with HIV and malaria infection in Port Harcourt, Nigeria. International Research Journal of Medical Sciences. 2016 Sep; 4(9):11-16.
- Fruman DA, Cantley LC. Idelalisib--a PI3Kδ inhibitor for B-cell cancers. N Engl J Med. 2014 Mar 13;370(11):1061-2. [Crossref] [Pubmed] [Pubmed Central]
- Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013 Apr;131(4):959-71. [Crossref] [Pubmed] Epub 2013 Mar 5.
- Goldberg BS, Ackerman ME. Antibody-mediated complement activation in pathology and protection. Immunol Cell Biol. 2020 Apr;98(4):305-317. [Crossref] [Pubmed] [Pubmed Central] Epub 2020 Apr 6.
- Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010 Feb;125(2 Suppl 2):S3-23. [Crossref] [Pubmed] [Pubmed Central]
- Chandra RK, Newberne PM. Interactions of Nutrition, Infection, and Immune Response in Animals. In: Nutrition, Immunity, and Infection. Chapter 7. New York: Plenum Press. 1977. [Crossref]
- Greensmith J, Whitbrook A, Aickelin U. Artificial Immune Systems. In: Gendreau M, Potvin JY (eds). Handbook of Metaheuristics. Chapter 14. Boston, MA: Springer. 2010. [Crossref]
- Yamada K, Hung P, Park TK, Park PJ, Lim BO. A comparison of the immunostimulatory effects of the medicinal herbs Echinacea, Ashwagandha and Brahmi. J Ethnopharmacol. 2011 Sep 1;137(1):231-5. [Crossref] [Pubmed] Epub 2011 May 17.
- Khan SR, van der Burgh AC, Peeters RP, van Hagen PM, Dalm VASH, Chaker L. Determinants of serum immunoglobulin levels: A systematic review and meta-analysis. Front Immunol. 2021 Apr 7;12:664526. [Crossref] [Pubmed] [Pubmed Central]
- Draborg A, Izarzugaza JM, Houen G. How compelling are the data for Epstein-Barr virus being a trigger for systemic lupus and other autoimmune diseases? Curr Opin Rheumatol. 2016 Jul;28(4):398-404. [Crossref] [Pubmed]
- Rai T, Wu X, Shen B. Frequency and risk factors of low immunoglobulin levels in patients with inflammatory bowel disease. Gastroenterol Rep (Oxf). 2015 May;3(2):115-21. [Crossref] [Pubmed] [Pubmed Central] Epub 2015 Jan 30.

