Exercise and the cytokines-interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α): A review
Exercise and the cytokines, IL-6, TNF-α
Accepted: 2017-04-07
Online: 2017-04-08
Print: 2017-04-30

Full text
Abstract
Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were one of the first few cytokines to be discovered. The normative data for levels of cytokines IL-6 and TNF-α in particular and all other cytokines in general have not yet been established well. The normal levels for each of the cytokines vary from one race to another. Therefore, all studies need to be done in cases and controls belonging to the same race or same populations. The kits for cytokine assays are expensive and running the assays is laborious and time consuming. It is recommended that the serum/plasma samples are run in duplicates and triplicates to avoid error. Immunology and the field of cytokines is an area which has many domains unexplored. As yet, it is not clearly understood by what mechanisms and pathways each of the cytokines alter the levels of other cytokines. Exercise or physical activity is an intervention which can be administered easily and levels of cytokines measured before and after intervention in same individuals taking all the above mentioned factors into consideration. Hence it is imperative that we look into studies on exercise and cytokines to do further research in the field of cytokines.
Keywords
Cytokines, Exercise, Interleukin-6, Tumor necrosis factor-α
Introduction
Cytokines are protein molecules which are mediators of our immune system [1] that are released into the circulation by several cells of the reticulo-endothelial and musculoskeletal systems [2] A variety of cytokines are pumped into the circulation in response to different stimuli such as antigens, exercise, mental stress, infections, etc [3].
Interleukin-6 and tumor necrosis factor-α
Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are two cytokines that are known to mediate changes in musculoskeletal and immune systems [4]. They are pro-inflammatory cytokines that mediate process of inflammation and healing [5]. However when released in excess, they can cause unwanted exacerbation of inflammation [6]. This is particularly harmful when there is a pre-existing low-grade inflammation as in several chronic diseases [7]. The crystal structures of the two cytokines as published in the protein data bank (PDB) are depicted in Fig 1 and Fig 2.

Functions of IL-6 and TNF-α
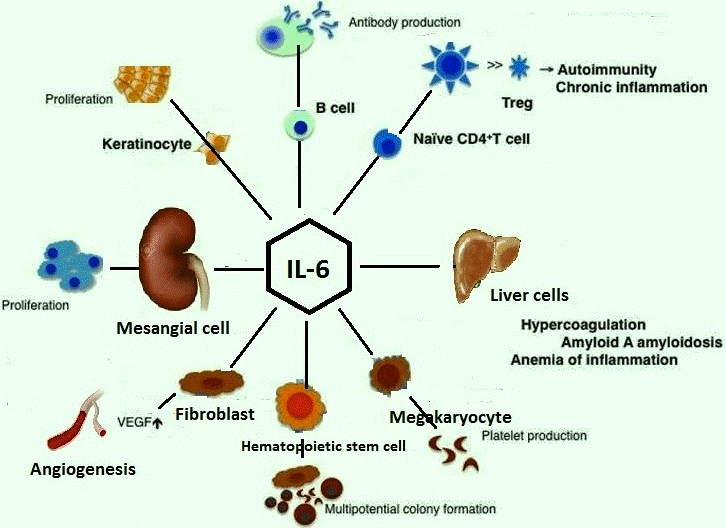
IL-6 stimulates terminal differentiation of B-lymphocytes and enhances immunoglobulin secretion by the B cells [8]. It is a cofactor for hemopoietic colony growth and thymocytes [9]. It stimulates hepatocytes to produce acute phase proteins like the C-reactive protein (CRP) [10]. It also acts as a growth factor for B cells [11].
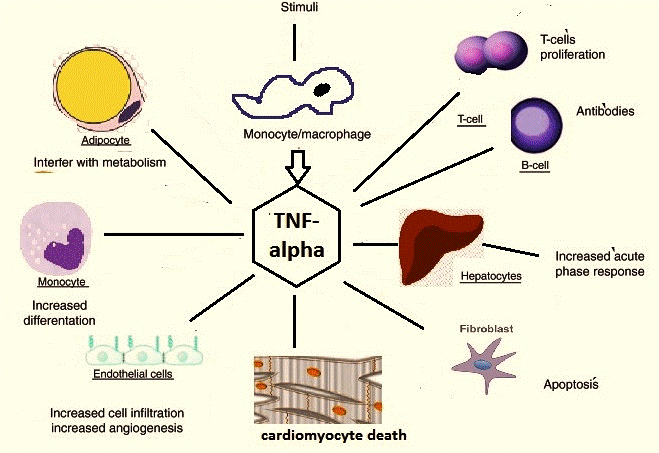
TNF-α is a multifunctional and a pleiotropic stimulator of cellular responses [12]. It is cytolytic to certain tumor cells [13]. It is an inducer of cytokines and cell adhesion molecules and is a regulator of proliferation/differentiation in lymphocytes and hemopoietic progenitors [14]. It is also known to cause insulin resistance [15].
The levels of these cytokines are altered with different types of exercise such as downhill running, cycling, swimming, athletics, etc. depending on the predominant type of the subsets of the T-lymphocytes (such as Th-1, Th-2, Th suppressor, T-regulator cells, etc.) present [16]. It has been postulated by researchers that the balance of cytokines in a particular tissue location or blood influences the differentiation of mature T-cell subsets from their precursors [17]. Few actions of IL-6 and TNF-α overlap with each other (Fig 3 and Fig 4).
IL-6 and TNF-α alterations with exercise in animal models
Ainsworth et al [18] studied the effect of strenuous exercise on horses and studied their profile of cytokine changes but the results of this study were equivocal. However, Xiang et al [19] showed that obese Zucker rats subjected to regular moderate exercise had lower IL-6 levels compared to obese sedentary rats. They also showed acetylcholine-induced vasodilatation was much more pronounced in the former group of rats. Keller et al [20] demonstrated that exercise normalizes over-expression of TNF-α in mice. He postulated that this effect might be mediated by IL-6. Faldt et al [21] suggested that IL-6 exerts its effect by stimulating energy expenditure and reducing body fat mass. Further, IL-6 deficient mice had reduced endurance and energy expenditure during exercise.
Behavior of IL-6 and TNF-α with exercises in humans
Scientists from different countries the world over have researched cytokine changes in humans, both in normal and diseased individuals. Varied modes of exercise have been used in a wide spectrum of studies.
Exercise and the cytokines in normal individuals
Unaccustomed eccentric exercise such as downhill running on a treadmill is shown to increase plasma IL-6 levels drastically [22]. Eccentric strenuous exercise causes IL-6 expression in myofibers preceding the disruption of the same myofibers. Thus an increased level of IL-6 is correlated with muscle damage caused by strenuous exercise [23]. Sustained, strenuous exercise as in military training programs induces immune impairment through decrease in mucosal immunity and also leads to release of excess IL-6 into circulation [24]. It has been pointed out that IL-6, the fatigue generating pro-inflammatory and pro-healing factor may participate in healing of tissue trauma when released in optimum quantities [25]. Therefore, moderate exercises done on a regular basis help in maintenance of optimum levels of IL-6 and hence result in good health [26,27].
IL-6 and TNF-α in diseases and disorders
Regular moderate exercise has also been shown to be beneficial in chronic diseases with low-grade inflammation [28]. Patients with such diseases have been shown to exhibit high levels of plasma IL-6 and TNF-α compared to normal individuals [29]. The inflammation can be brought down and the diseases controlled by regular exercise and dietary weight loss; after which levels of IL-6 and TNF-α decrease in the plasma. Bouts of unaccustomed exercise in the same patients are known to increase IL-6 and TNF-α levels in plasma and exacerbate the disease process 30].
Exercise and pro-inflammatory cytokines are double edged swords
IL-6 and TNF-α levels is seen to fall after appropriate and optimum exercise regimes in patients of type 2 diabetes mellitus and cardiovascular diseases, thus leading to improvement in health status. TNF-α is known to cause insulin resistance. Therefore exercise also increases insulin sensitivity by bringing down TNF-α levels. IL-6 has also been called as myokine or exercise factor or work factor by various authors. Maintenance of optimum levels of IL-6 in particular, by regular exercise, can result in protection against chronic diseases associated with low-grade inflammation such as diabetes mellitus and cardiovascular diseases [31,32]. Coronary risk profile is seen to improve in coronary artery disease (CAD) patients after regular exercise regime due to a decrease in C-reactive protein (CRP), IL-6, TNF-α, Interleukin-1 (IL-1) and Interferon gamma (IFN-γ) [33]. Studies done on healthy older males also showed decreased IL-6 levels after regular exercise regime. Thus exercise may play a vital role in controlling inflammatory markers during the aging process [34]. A drastic decrease in IL-6 is associated with decreased exercise capacity [35]. However, acute IL-6 administration impairs athletic performance in healthy trained male runners, so every individual needs to maintain an optimum level of IL-6 concentration according to his or her health profile [36]. An excess of IL-6 and TNF-α are also shown to induce skeletal muscle atrophy [29,37]. IL-6 that is produced by the muscle during exercise is shown to have many biological roles such as induction of lipolysis, suppression of TNF-α production and stimulation of cortisol production [38]. Apart from physical stress, psychological stress also influences the immune system by altering the levels of pro-inflammatory cytokines [39].
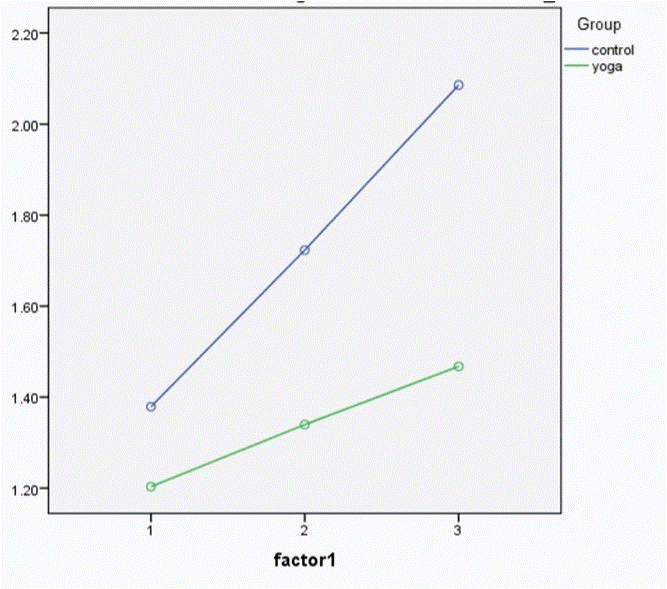
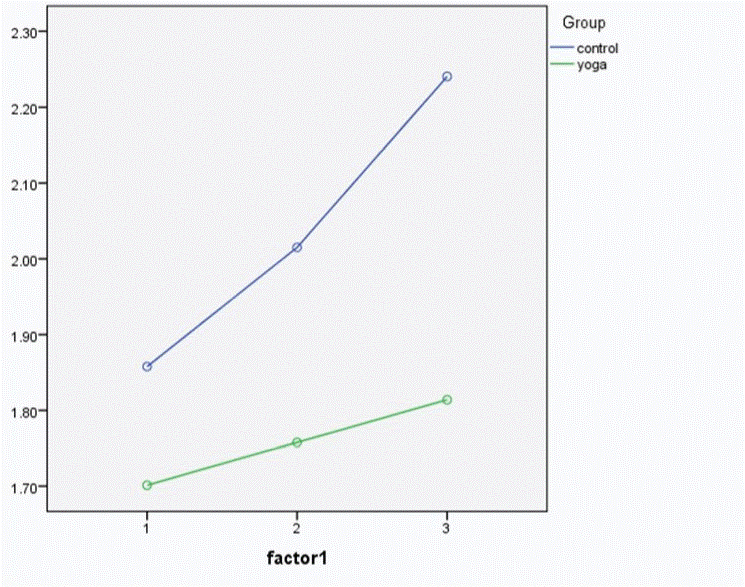
The authors of the article have found that both IL-6 and TNF-α increase with a bout of exercise of moderate intensity and further increased with a bout of strenuous exercise. The levels of both cytokines were lower in long term practitioners of Yoga when compared to controls. Further, the rate of increase in levels of pro-inflammatory cytokines was less in Yoga group compared to controls, probably indicating that regular practice of yoga cushions the effect of physical stress on our immune system and protects the body against unnecessary inflammation [40]. (Fig 5 and Fig 6)
Acknowledgments
We, the authors acknowledge the contribution of Dr. Arun Kumar M., Assistant Professor of Physiology, M. S. Ramaiah Medical College for his illustrative work in producing figures 3 and 4 as per the authors' requirements.
Source of funding
None
Conflict of interest
The author declare that no competing interests exist.
References
- Wikipedia. Cytokine. Available from https://en.wikipedia.org/w/index.php?title=Cytokine&oldid=766745372 [Last accessed April 2, 2017]
- Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013; 2013:434010. [Pubmed][Crossref]
- Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011 Jul 7; 118(1):9-18. [Pubmed][Crossref]
- Machado JR, Soave DF, da Silva MV, de Menezes LB, Etchebehere RM, Monteiro ML, dos Reis MA, Corrêa RR, Celes MR. Neonatal sepsis and inflammatory mediators. Mediators Inflamm. 2014; 2014:269681. [Pubmed][Crossref]
- Pandiyan P, Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine. 2015 Nov; 76(1):13-24. [Pubmed][Crossref]
- Spoto B, Di Betta E, Mattace-Raso F, Sijbrands E, Vilardi A, Parlongo RM, Pizzini P, Pisano A, Vermi W, Testa A, Cutrupi S, D'Arrigo G, Lonardi S, Tripepi G, Cancarini G, Zoccali C. Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr Metab Cardiovasc Dis. 2014 Oct; 24(10):1137-43. [Pubmed][Crossref]
- Siebert S, Tsoukas A, Robertson J, McInnes I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol Rev. 2015; 67(2):280-309. [Pubmed][Crossref]
- Mauer J, Denson JL, Brüning JC. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015 Feb;36(2):92–101. [Pubmed][Crossref]
- Platel D, Guiguet M, Briere F, Bernard A, Mack G. Human interleukin-6 acts as a co-factor for the up-regulation of C3 production by rat liver epithelial cells. Eur Cytokine Netw. 1994 Jul-Aug; 5(4):405-10. [Pubmed]
- Zupke CA, Stefanovich P, Berthiaume F, Yarmush ML. Metabolic effects of stress mediators on cultured hepatocytes. Biotechnol Bioeng. 1998 Apr 20-May 5; 58(2-3):222-30. [Pubmed]
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015 May; 16(5):448-57. [Pubmed][Crossref]
- Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016 Jan; 12(1):49-62. [Pubmed][Crossref]
- Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006 Sep; 25(3):409-16. [Pubmed][Crossref]
- Dajani R, Sanlioglu S, Zhang Y, Li Q, Monick MM, Lazartigues E, Eggleston T, Davisson RL, Hunninghake GW, Engelhardt JF. Pleiotropic functions of TNF-alpha determine distinct IKKbeta-dependent hepatocellular fates in response to LPS. Am J Physiol Gastrointest Liver Physiol. 2007 Jan; 292(1):G242-52. [Pubmed][Crossref]
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000 Aug;11(6):212-7. [Pubmed][Crossref]
- Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011; 17: 6-63. [Pubmed]
- Moudgil KD. Interplay among cytokines and T cell subsets in the progression and control of immune-mediated diseases. Cytokine. 2015 Jul; 74(1):1-4. [Pubmed][Crossref]
- Ainsworth DM, Appleton JA, Eicker SW, Luce R, Julia Flaminio M, Antczak DF. The effect of strenuous exercise on mRNA concentrations of interleukin-12, interferon-gamma and interleukin-4 in equine pulmonary and peripheral blood mononuclear cells. Vet Immunol Immunopathol 2003 Jan 10; 91(1):61-71. [Pubmed][Crossref]
- Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005 Apr; 288(4):R987-91. [Pubmed][Crossref]
- Keller C, Keller P, Giralt M, Hidalgo J, Pedersen BK. Exercise normalises overexpression of TNF-alpha in knockout mice. Biochem Biophys Res Commun 2004 Aug 13; 321(1):179-82. [Pubmed][Crossref]
- Faldt J, Wernstedt I, Fitzgerald SM, Wallenius K, Bergstrom G, Jansson JO. Reduced exercise endurance in interleukin-6-deficient mice. Endocrinology 2004 Jun; 145(6):2680-6. [Pubmed][Crossref]
- Peake JM, Suzuki K, Wilson G, Hordern M, Nosaka K, Mackinnon L, Coombes JS. Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activation. Med Sci Sports Exerc. 2005 May; 37(5):737-45. [Pubmed]
- Tomiya A, Aizawa T, Nagatomi R, Sensui H, Kokubun S. Myofibers express IL-6 after eccentric exercise. Am J Sports Med 2004 Mar; 32(2):503-8. [Pubmed][Crossref]
- Gomez MD, Chennaoui M, Burnat P, Drogou C, Guezennec CY. Immune and hormonal changes following intense military training. Mil Med 2003 Dec; 168(12):1034-8. [Pubmed]
- Steensberg A. The role of IL-6 in exercise-induced immune changes and metabolism. Exerc Immunol Rev 2003; 9:40-7. [Pubmed]
- Filteau SM, Menzies RA, Kaido TJ, O’Grady MP, Gelderd JB, Hall NR. Effects of exercise on immune functions of undernourished mice. Life Sci 1992; 51(8):565-74. [Pubmed]
- Blair SN, Kohl HW, Gordon NF, Paffenbarger RS Jr. How much physical activity is good for health? Annu Rev Public Health 1992; 13:99-126. [Pubmed][Crossref]
- Shepard RJ, Shek PN. Autoimmune disorders, physical activity, and training, with particular reference to rheumatoid arthritis. Exerc Immunol Rev 1997; 3:53-67. [Pubmed]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014 Jun; 69 Suppl 1:S4–9. [Pubmed][Crossref]
- Nicklas BJ, You T, Pahor M. Behavioral treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ 2005 Apr 26; 172(9):1199-209. [Pubmed][Crossref]
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005 Apr; 98(4):1154-62. [Pubmed][Crossref]
- Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 2004 Jul; 53(7):1643-8. [Pubmed][Crossref]
- Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005 Apr 8; 100(1):93-9. [Pubmed][Crossref]
- Jankord R, Jemiolo B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med Sci Sports Exerc. 2004 Jun; 36(6):960-4. [Pubmed]
- Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Wolsk-Petersen E, Febbraio M. The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? Proc Nutr Soc. 2004 May; 63(2):263-7. [Pubmed][Crossref]
- Robson-Ansley PJ, de Milander L, Collins M, Noakes TD. Acute interleukin-6 administration impairs athletic performance in healthy, trained male runners. Can J Appl Physiol. 2004 Aug; 29(4):411-8. [Pubmed]
- Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985). 2005 Mar; 98(3):911-7. [Pubmed][Crossref]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008 Oct; 88(4):1379-406. [Pubmed][Crossref]
- Tomei LD, Kiecolt-Glaser JK, Kennedy S, Glaser R. Psychological stress and phorbol ester inhibition of radiation-induced apoptosis in human peripheral blood leukocytes. Psychiatry Res. 1990 Jul; 33(1):59-71. [Pubmed]
- Vijayaraghava A, Doreswamy V, Narasipur OS, Kunnavil R, Srinivasamurthy N. Effect of yoga practice on levels of inflammatory markers after moderate and strenuous exercise. J Clin Diagn Res. 2015 Jun; 9(6):CC08-12. [Pubmed][Crossref]

