Slow breathing has unequal effects on prehypertensives from different ethnic/racial groups
Effect of slow breathing in different ethnic groups
Accepted: 2017-03-18
Online: 2017-03-28
Print: 2017-04-30

Full text
Abstract
In this study, we investigated whether slow breathing reduces blood pressure (BP) in individuals at risk of developing hypertension and if slow breathing has the same effect on Caucasian, African, Arabian and Indian subjects. Also we assessed ethnic/racial differences in low frequency (LF) power and high frequency (HF) power of heart rate variability (HRV). A total of 40 Caucasian men from Ukraine, 39 West African men mostly from Nigeria, 38 Arabic men from Palestine and Israel and 41 South Asian men from India studying at V. N. Karazin Kharkiv National University were recruited in this study. The subjects were further classified into normotensive and prehypertensive groups. Heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), LF power, HF power of HRV were recorded at spontaneous breathing and at paced breathing of 10 and 6 breaths per minute. It was found that slowing respiratory rate to 6 breaths per minute reduces SBP in prehypertensive Caucasians, Arabs, Indians, but not in Africans. At 6 breaths per minute, natural logarithm of HF (LnHF) power indicating cardiovagal activity was less in normotensive Arabs than in Caucasians, Africans and Indians possibly suggesting an increased risk of developing hypertension; while prehypertensive Africans demonstrated LnHF power higher than Arabs and Indians. When covariates like age and body mass index (BMI) were considered, prehypertensive Africans demonstrated LnHF power higher than in Caucasians also. It is suggested that in prehypertensive Africans the control of autonomic nervous activity is reset to a higher level of parasympathetic outflow.
Keywords
Blood pressure, Ethnicity, Heart rate variability, Prehypertension, Race
Introduction
A dysregulation of the autonomic nervous system (ANS) causes loss of homeostasis, leads to pathology and underlies virtually every disease, including hypertension. Therefore, assessment of autonomic nervous system activity is vital for healthy subjects[1] and in individuals developing hypertension [2,3,4,5,6,7]. Ethnic/Racial differences in predisposition to hypertension are widely documented in the literature showing high prevalence of hypertension in African-Americans, black Africans, and Arabs [2,3,4,5,6,7]. However, data regarding the prevalence of hypertension in South Asians are controversial, unlike the results concerning to the high prevalence of coronary heart disease (CHD) in this population [8]. It has been demonstrated that slowing breathing to 6 breaths per minute can reduce blood pressure (BP) in hypertensive patients, patients with chronic heart failure, and patients with chronic obstructive pulmonary disease, but not in normotensive control subjects [9,10,11]. The mechanism through which the reduction of respiratory frequency decreases blood pressure may be associated with the reduction of sympathetic traffic by enhancing central inhibitory rhythms or/and enhancing the baroreflex. However, prevention is better than cure, and it remains unclear whether slow breathing affects blood pressure (BP) in subjects developing hypertension in different ethnic/racial populations.
Currently, heart rate variability (HRV) is a widely used tool for the evaluation of ANS contribution in the cardiac function in health and disease [12,13,14]. The spectral power of high frequency (HF) of HRV oscillations is widely accepted as a measure of the cardiac vagal activity, meanwhile an interpretation of the spectral power of low frequency (LF) is more controversial [12,13,14].
In the present study we investigated whether the slow breathing reduces blood pressure in individuals developing hypertension and if the slow breathing has the same effect on Caucasian, African, Arabic, and Indian subjects. The second aim was to test for possible effect of ethnicity/race on HRV in normotensive and prehypertensive subjects at spontaneous and slow breathing.
Materials and Methods
Subjects
Participant cohort included 158 volunteers recruited from the student population of the V.N. Karazin Kharkiv National University: 40 Caucasian men (age: 19.8±0.3 years, BMI: 23.4±0.6 kg/m2) from Ukraine; 39 black West African men (age: 20.4±0.3 years, BMI: 22.9±0.4 kg/m2) mostly from Nigeria; 38 Arabic men (age: 20.4±0.2 years, BMI: 23.8±0.6 kg/m2) from Palestine and Israel; 41 South Asian men (age: 19.8±0.3 years, BMI: 22.9±0.6 kg/m2) from India. There were no significant differences between the groups in age, height, weight, and BMI. The subjects were classified into two groups based on their level of systolic blood pressure (SBP) as following. The inclusion criteria for the first group: SBP < 125 mm Hg, and for the second group: SBP ≥ 125mm Hg. The first group as a total, included individuals with 116.9 – 118.7 mm Hg 95% confidence interval (95% CI) for SBP, according to JNC-7 classification[15], referred thereafter to as the normotensive group. The 95% CI for SBP in the second group was 130.0 – 132.5 mm Hg, referred thereafter to as the prehypertensive group. The diastolic blood pressure (DBP) was in the normal range in the both groups, 95% CI for DBP was 67.6 – 69.4 mm Hg and 75.0 – 77.6 mm Hg in the normotensive and prehypertensive group, respectively. No one prehypertensive subject had ever been treated for hypertension. Subjects with cardiac rhythm abnormalities, respiratory, or metabolic diseases were excluded from the study.
All procedures were in accordance with the ethical standards of the Ethics Committee of the Biological Department of V.N. Karazin Kharkiv National University and with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was obtained from all participants included in the study.
Study design
After stabilization the participants were allowed to breathe spontaneously in the supine position during baseline stage, followed by 0.17-Hz (10 breaths per minute) slow breathing stage, during which the participants were instructed to control their breathing according to the audio signals generated by paced breathing test software for HRV analysis (CardioLab CS, XAI-Medica, Ukraine)[16] and to breathe as comfortably and effortlessly as possible. The durations of the inspiratory and expiratory phases were set at 1:1. The next test, composed of the spontaneous breathing stage and 0.10-Hz (6 breaths per minute) slow breathing, was separated from previous one by 30 min interval. All subjects have undergone four stages each of 5 min duration.
HRV and blood pressure (BP) measurements
ECG from three standard leads was continuously recorded using a digital 12-channel ECG amplifier with a sampling frequency 1000 Hz (CardioLab 2010, XAI-Medica, Ukraine)[16]. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were determined using the automatic blood pressure digital sphygmomanometer (Nissei WS-1011, Nihon Seimitsu Sokki Co., Ltd, Japan). Blood pressure was measured at 4.5 minute of each of the four stages. Since HR, BP, HRV indices were recorded twice at spontaneous breathing, the average of two measurements was used in the analysis.
Heart rate variability was assessed in the frequency-domain analysis by means of a nonparametric method of fast Fourier transformation. Interpreting spectral power, we proceeded from the assumption that HF power received from high frequency oscillations ranging from 0.15 to 0.40 Hz is a measure of the cardiovagal outflow [12,13,14], and LF power computed from low frequency oscillations ranging from 0.04 to 0.15 Hz is the baroreflex-mediated index of cardiac autonomic regulation [13].
Statistical analysis
Data are presented as means±SE. Three-way repeated measures MANOVA with Ethnicity/Race and HT as between-groups independent variables and breathing frequency as a repeated-measures within-groups independent variable was used to test for possible effects of ethnicity/race, HT, breathing frequency, and their interaction on HR, SBP, DBP, LnHF power, LnLF power. To meet the repeated measures MANOVA assumptions the outcome variables that were not normally distributed were naturally log-transformed (LF power and HF power). Three-way repeated measures MANCOVA was used to test for possible ethnicity/race, blood pressure category, and breathing frequency effects after adjusting for age and BMI. The simple effects were tested by means SPSS command syntax with Bonferroni correction. Statistical analysis was performed with the SPSS statistical program, version 22.
Results
Effect of slow breathing on heart rate
Slowing respiratory rate to 10 breaths per minute led to a significant increase in HR (HR10) compared with spontaneous breathing (HRsp) in all the normotensive and prehypertensive groups with the exception of prehypertensive Africans (Table 1). However, slowing respiratory rate to 6 breaths per minute led to a significant increase in HR (HR6) in all the prehypertensive groups and in normotensive Indians, but not in the other normotensive groups.
At respiratory rate of 10 breaths per minute HR was significantly less in prehypertensive Africans than in prehypertensive Arabic and Indian groups (Table 1).
There were no significant differences in HR between normotensives and prehypertensives.
| Normotensive | Prehypertensive | |||||||
|---|---|---|---|---|---|---|---|---|
| Caucasian | African | Arabian | Indian | Caucasian | African | Arabian | Indian | |
| n | 21 | 17 | 21 | 26 | 19 | 22 | 17 | 15 |
| HR (bpm) | ||||||||
| HRsp | 63.7±1.9 | 64.1±1.6 | 68.5±2.0 | 67.5±2.3 | 67.6±2.1 | 63.4±1.4 | 69.9±2.1 | 70.3±2.4 |
| HR10 | 67.1±1.7* | 69.4±2.1*‡ | 74.2±2.3*‡ | 73.3±2.2* | 72.9±2.4* | 65.4±1.7 | 75.8±2.6*‡b | 74.7±3.1*b |
| HR6 | 65.5±1.6 | 65.6±1.9 | 70.3±1.8 | 70.4±2.2* | 70.4±2.4* | 67.5±1.7* | 72.2±2.4* | 72.7±2.9 |
| SBP (mm Hg)# | ||||||||
| SBPsp | 119.0±0.8 | 119.4±1.0 | 117.8±0.8 | 115.9±0.9† | 129.6±1.0‡§ | 131.4±1.1§ | 133.6±1.6‡§ | 130.4±1.0‡§ |
| SBP10 | 118.1±1.5d | 118.8±1.1d | 116.1±1.4 | 112.5±1.2 | 127.2±1.8§ | 129.4±1.7§ | 130.4±2.0§ | 128.2±1.5§ |
| SBP6 | 119.0±2.0 | 118.4±2.0 | 116.6±1.2 | 114.4±1.4 | 124.4±1.3§ | 128.8±2.0§ | 129.5±2.5§ | 126.0±1.4§ |
| DBP (mm Hg) | ||||||||
| DBPsp | 68.1±1.0 | 69.9±1.2 | 66.8±0.7 | 69.2±0.8 | 74.3±1.1§ | 76.4±1.2§ | 76.0±1.2§ | 79.0±1.5a§ |
| DBP10 | 68.4±1.0 | 70.8±1.4 | 67.8±1.1 | 68.0±0.9 | 73.5±1.1§ | 77.3±1.7§ | 76.2±1.1§ | 78.5±1.6§ |
| DBP6 | 67.1±1.4 | 71.0±1.8 | 66.5±1.2 | 68.1±1.0 | 73.5±0.9§ | 77.2±1.4§ | 76.2±1.8§ | 77.1±1.6§ |
| LnLF (ms2) | ||||||||
| LnLFsp | 7.08±0.13 | 6.72±0.19 | 6.86±0.18† | 6.81±0.15 | 7.39±0.17† | 7.00±0.20 | 6.97±0.16 | 7.07±0.19 |
| LnLF10 | 6.88±0.18 | 6.34±0.25 | 6.33±0.20 | 6.82±0.20 | 6.56±0.17 | 6.96±0.22 | 6.61±0.17 | 6.72±0.25 |
| LnLF6 | 8.96±0.14*† | 8.89±0.14*† | 8.49±0.16*† | 8.74±0.10*† | 8.77±0.14*† | 9.02±0.13*† | 8.57±0.19*† | 8.63±0.14*† |
Data are expressed as means±SE; n=number of participants; *P<0.05, vs. resting indices; †P<0.05, vs. indices measured at 10 breaths per minute; ‡P<0.05, vs. indices measured at 6 breaths per minute; §P<0.05 vs normotensive counterparts; aP<0.05, vs. prehypertensive Caucasians; bP<0.05, vs. prehypertensive Africans; cP<0.05, vs. Arabs with similar blood pressure; dP<0.05, vs. Indians with similar blood pressure; #P<0.05 significant breathing frequency by HT interaction were found from general linear model (GLM) three-way repeated measures MANOVA with Bonferroni correction for multiple comparisons. |
||||||||
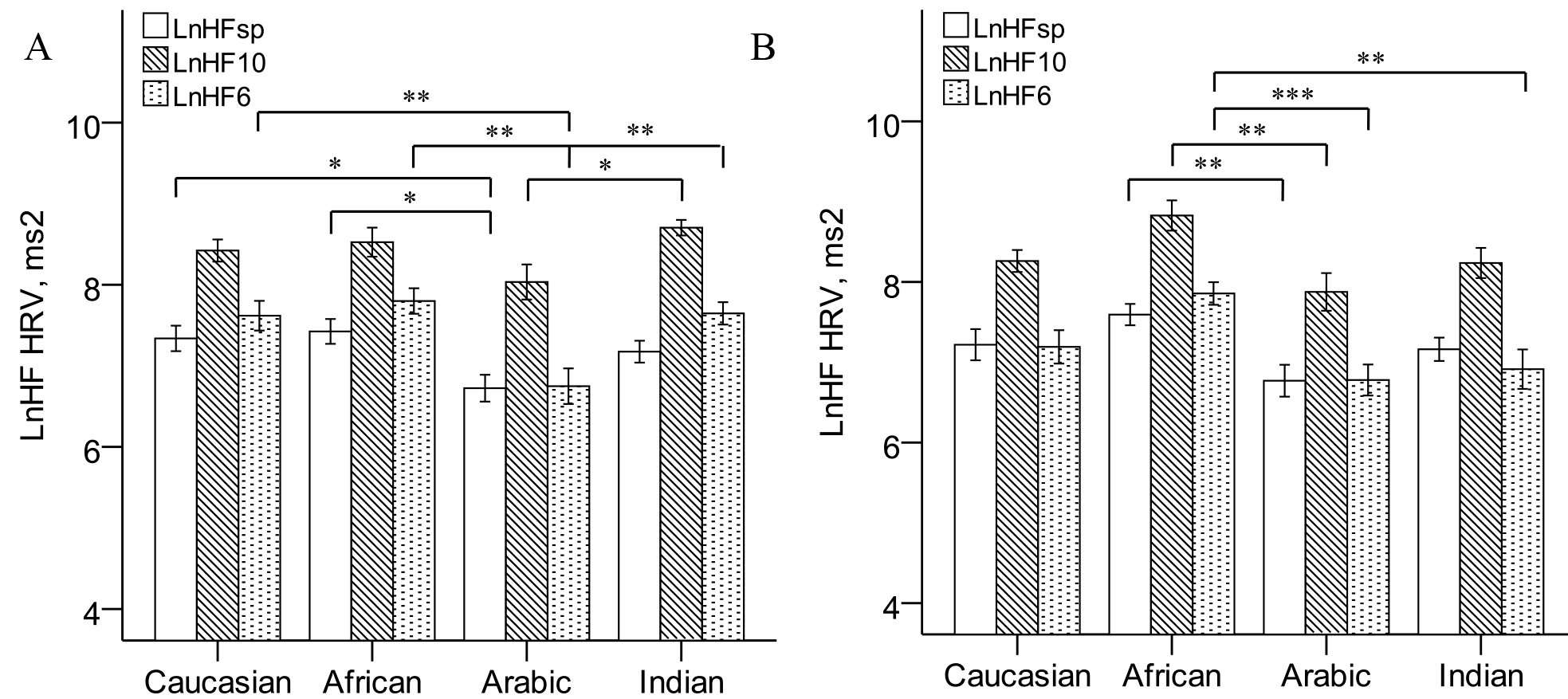
Effect of slow breathing on blood pressure
Slowing breathing to 10 breaths per minute led to a significant reduction in SBP (SBP10) compared with that at spontaneous breathing (SBPsp) in normotensive Indians only (Table 1). However, paced breathing at 6 breaths per minute reduced SBP (SBP6) only in prehypertensives, with the exception of Africans demonstrating the similar trend but without significant differences (Table 1).
Unlike systolic blood pressure, the diastolic blood pressure did not change significantly with the respiratory rate variations in either normotensive or prehypertensive group at any respiratory rate of slow breathing (Table 1).
The normotensive Indian group demonstrated SBP less than normotensive Caucasians and Africans at the respiratory rate of 10 breaths per minute and prehypertensive Indians had DBP significantly higher than prehypertensive Caucasian group at spontaneous breathing (DBPsp) (Table 1).
Effect of paced breathing on LF power and HF power
At 6 breaths per minute LnLF power (LnLF6) was significantly higher in all the groups compared with that at spontaneous breathing (LnLFsp) and at 10 breaths per minute (LnLF10). In addition, in normotensive Arabs and prehypertensive Caucasians LnLF10 was significantly less than LnLFsp (Table 1). No ethnic/racial differences in LnLF power were found at spontaneous and paced breathing.
Similarly, slowing breathing frequency to 10 breaths per minute led to a significant increase in LnHF power (LnHF10) in normotensive (Fig 1) and prehypertensive (Fig 1) groups.
In the normotensive Arabic group LnHFsp was less than in normotensive Caucasians and Africans, LnHF10 was less than in Indians, and LnHF6 was less than in all the other normotensive groups at high significant level (Fig 1). Prehypertensive Africans had LnHFsp and LnHF10 higher than prehypertensive Arabs (Fig 1). However, LnHF6 in prehypertensive Africans was higher compared with Arabic and Indian men (Fig 1). When covarying for age and BMI, compared with Caucasians also (for LnHF6 adjusted means: 7.88±0.18 vs. 7.17±0.19 ms2, respectively, P=0.043, not shown).
There were no significant differences between normotensive and prehypertensive counterparts in LnLF and LnHF power.
Discussion
Effect of paced breathing on HF and LF power
In the current study we proceeded from the widely accepted assumption that HF HRV power reflects cardiovagal activity [12,13,14] and from the hypothesis that that LF HRV power is a measure of baroreflex-mediated regulation of the autonomic outflow[13]. In agreement with previous studies we have demonstrated that the slow breathing greatly affected both HF power and LF power, such that slow breathing at respiratory rate of 6 breaths per minute significantly increased LF power while slow breathing at 10 breaths per minute largely increased HF power [17,18,19] in all the groups. So, according to ours and previous studies, it is obvious that respiration not only is the major contributor to the genesis of the HF peak in the HRV power spectrum indicating cardiovagal outflow, but also strongly influences LF power, possibly reflecting the baroreflex-mediated regulation of cardiac autonomic outflows [13] at respiratory rate of 6 breaths per minute and/or sympathetic outflow[17,18] contributing into respiratory sinus arrhythmia.
Effect of paced breathing on HR
The current findings indicated a significant increase in HR in all the groups (with the exception of the prehypertensive Africans) at 10 breaths per minute and in all the prehypertensive groups at 6 breaths per minute. These data are in partial agreement with previous studies in that slowing of breathing frequency to 6 breaths per minute did not influence significantly HR in normotensive subjects [9,10,18,19] and has a greater chronotropic effect in hypertensive individuals [17]. However, the significant increase in HR in hypertensive subjects at 6 breaths per minute was not observed in previous reports [9]. Similarly, previous studies did not report a significance increase in HR in either normotensive [18,19] or hypertensive group at 10 breaths per minute found in the current study. The conflicting results may be attributed to the different stages of hypertension, age, position, technique of respiration modulation. On our knowledge, effect of slow breathing was not evaluated in prehypertensives, moreover in the light of ethnic/racial differences.
An increase in HR despite a marked increase in HF power representing cardiovagal outflow in response to slowing breathing to 10 breaths per minute may be explained partially by the fact that the LnHF power reflected cardiovagal variations within the respiratory cycle but does not alter the net tonic level of parasympathetic outflow [19]. Similarly, an increased HR in prehypertensives at 6 breaths per minute and the markedly increased LF power, possibly indicates that LF power reflects distribution of the baroreflex modulation and/or sympathetic influences within the various phases of the respiratory cycle [17,18,19].
Effect of slow breathing on BP
Our data have indicated that paced breathing at 10 breaths per minute influence SBP in neither normotensive nor prehypertensive groups (except normotensive Indians). However, slowing breathing rate to 6 breaths per minute led to a significant reduction in SBP in prehypertensive groups with the exception of Africans. These findings are in agreement with those in previous studies, indicating a significant effect of slow breathing on SBP in hypertensive patients [9], but not in normotensive subjects [9,10,18]. Taking into account the hypothesis indicating that LF power is a measure of baroreflex-mediated modulation of cardiac autonomic outflows [13] and also data from previous studies demonstrating that slow breathing improves baroreflex sensitivity in both normotensives and hypertensives, but reduces sympathetic outflow in hypertensive patients only [9] it is suggested that the increase in LF power and, possibly, baroreflex sensitivity led to the reduction in SBP in prehypertensive subjects.
Ethnic/Racial differences in hemodynamic and HRV indices
Several studies found statistically significant association between high risk of incident hypertension and low HF power [20,21] indicating that autonomic disregulation is present in the early stage of hypertension or even precedes the development of clinical hypertension. Despite a high prevalence of hypertension among African-Americans and Black Africans [2,3,4,5], several studies indicated that people from African descent have greater vagal activity compared with people from European descent, as estimated by means HRV analysis [2,16,22]. In agreement with these reports the current data revealed that at 6 breaths per minute LnHF power was significantly higher in prehypertensive Africans than in Arabic and in Indian prehypertensive subjects, and when covarying for age and BMI, in prehypertensive Caucasians also. At the same time, the only prehypertensive group that did not show significant reduction in SBP at 6 breaths per minute was the African group. These findings are similar to our previous study, reporting higher LnHF power in African men compared with Arabs only at supine rest, but with Caucasians, Arabs, and Indians in upright position under condition of the decreased venous return [16]. So, the higher cardiovagal activity found at slow breathing in Africans developing hypertension is not associated with BP and does not contribute to the high prevalence of hypertension in Africans.
The normotensive Arabs had LnHF power significantly lower than Africans at spontaneous breathing, lower than Indians at 10 breaths per minute, and lower than Caucasians, Africans, and Indians, at 6 breaths per minute. Little is known about predisposition of hypertension among Arabs; however, a few studies reported that hypertension is prevalent in Arabic population [6,7]. Previously, it was shown, that Arabs had enhanced pressor response to orthostasis, despite similar baseline BP among Caucasians, Africans and Indians [16]. Possibly, the low cardiovagal activity found in the current study in normotensive Arabs may be relevant to their predisposition to hypertension.
The current findings indicated that the normotensive Indians was the only group demonstrating a significant reduction of blood pressure at 10 breaths per minute and the only normotensive group in which HR increased at 6 breaths per minute compared with spontaneous breathing. In addition, in consistence with previous reports[8,16] indicating high DBP, low resting SBP and pulse pressure in Indians, it was shown that at spontaneous breathing in prehypertensive Indians DBP was higher than in Caucasians, and at 10 breaths per minute SBP was lower in Indians than in Caucasians and in Africans. Since, in agreement with previous studies we did not find significant differences in HRV indices between Indians and Caucasians and between Indian and African subjects [16,23], the differences in the response to slow breathing and blood pressure cannot be attributed to altered autonomic regulation, but possibly might be related to reduced capillary density, or, rarefaction, reported for healthy normotensive individuals of South Asian origin [24,25]. Further study of this population would elucidate this matter.
Conclusion
In summary, it was found that slowing respiratory rate to 6 breaths per minute reduces SBP in developing hypertension Caucasian, Arabic, and Indian subjects but not in Africans. At 6 breaths per minute, prehypertensive Africans demonstrated cardiovagal outflow higher than in prehypertensive Arabs and Indians, when covarying for age and BMI, higher than in Caucasians also, suggesting that the control of autonomic nervous activity is reset in Africans to a higher level of parasympathetic outflow. At the same time, in normotensive Arabs cardiovagal outflow was less than in normotensive Caucasians, Africans, and Indians, possibly suggesting an increased risk of developing hypertension.
Limitations of the study
A limitation of the study is that the physical activity of participants' daily life was not accounted for the interpreting the effect of slow breathing on the change in SBP and DBP. Although accounting for Physical Activity of (daily) Life Index is ideal, all subjects were recruited from the student population of the V. N. Karazin Kharkiv University, they were of the similar age, had similar education level and lifestyle. In addition, BMI as a rude surrogate of physical activity was taken into account and used a covariate.
Acknowledgments
Thanks to my mentors Full Professor Bondarenko VA and Shchyrova LS who delicately and carefully guided my scientific and educational researches; my colleagues Zabrodskiy RF and Chugay TA for technical assistance; the research participants for their enthusiastic participation, time and patience with this study.
Source of funding
None
Conflict of interest
None declared
References
- Ogunlade O, Ayoka AO, Akintomide A, Akomolafe RO, Akinsomisoye OS, Oyebola DO. Non-invasive assessment of cardiac autonomic functions in healthy young adults in Ile-Ife, South-Western Nigeria. Int J Clin Cardiol. 2015 Jun; 2:3. [Crossref]
- Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005 Mar 15; 111(10):1233–41. [Pubmed] [Crossref]
- Hill LK, Hu DD, Koenig J, Sollers JJ 3rd, Kapuku G, Wang X, Snieder H, Thayer JF. Ethnic differences in resting heart rate variability: a systematic review and meta-analysis. Psychosom Med. 2015 Jan; 77(1):16–25. [Pubmed] [Crossref]
- Onwuchekwa AC, Mezie-Okoye MM, Babatunde S. Prevalence of hypertension in Kegbara-Dere, a rural community in the Niger Delta region, Nigeria. Ethn Dis. 2012 Summer; 22(3):340–6. [Pubmed]
- Adeloye D, Basquill C, Aderemi AV, Thompson JY, Obi FA. An estimate of the prevalence of hypertension in Nigeria: a systematic review and meta-analysis. J Hypertens. 2015 Feb; 33(2):230–42. [Pubmed] [Crossref]
- Khdour MR, Hallak HO, Shaeen M, Jarab AS, Al-Shahed QN. Prevalence, awareness, treatment and control of hypertension in the Palestinian population. J Hum Hypertens. 2013 Oct; 27(10): 623–8. [Pubmed] [Crossref]
- Tailakh A, Evangelista LS, Mentes JC, Pike NA, Phillips LR, Morisky DE. Hypertension prevalence, awareness, and control in Arab countries: A systematic review. Nurs Health Sci. 2014 Mar; 16(1):126–30. [Pubmed] [Crossref]
- Agyemang C, Bhopal RS. Hypertension and coronary heart disease in South Asians. In: Patel KCR, Bhopal RS (eds). The epidemic of coronary heart disease in south Asian populations: causes and consequences, 1st ed, South Asian Health Foundation, pp.110-120, 2004.
- Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, Bernardi L. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005 Oct; 46(4):714–8. [Pubmed] [Crossref]
- Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, Frey AW, Yeung LY, Sanderson JE, Pedretti R, Tramarin R. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation. 2002 Jan; 105(2):143–5. [Pubmed] [Crossref]
- Raupach T, Bahr F, Herrmann P, Luethje L, Heusser K, Hasenfuss G, Bernardi L, Andreas S. Slow breathing reduces sympathoexcitation in COPD. Eur Respir J. 2008 Aug; 32(2):387–92. [Pubmed] [Crossref]
- Task Force of the European Society of Cardiology, and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996 Mar; 17(3):354–81. [Pubmed] [Crossref]
- Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011 Dec; 96(12):1255–61. [Pubmed] [Crossref]
- Reyes Del Paso GA, Langewitz W, Mulder LJ, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiol. 2013 May; 50(5):477–87. [Pubmed] [Crossref]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003 Dec; 42(6):1206–52. [Pubmed] [Crossref]
- Shekh VE. Sex and ethnic/racial differences in blood pressure and heart rate variability during orthostatic testing in young healthy individuals. J Phys Pharm Adv. 2016 Mar; 6(3):846–59. [Crossref]
- Novak V, Novak P, de Champlain J, Nadeau R. Altered cardiorespiratory transfer in hypertension. Hypertension. 1994 Jan; 23(1):104–13. [Pubmed] [Crossref]
- Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol (1985). 1993 Nov; 75(5):2310–7. [Pubmed]
- Pitzalis MV, Mastropasqua F, Massari F, Passantino A, Colombo R, Mannarini A, Forleo C, Rizzon P. Effect of respiratory rate on the relationships between RR interval and systolic blood pressure fluctuations: a frequency-dependent phenomenon. Cardiovasc Res. 1998 May; 38(2):332–9. [Pubmed] [Crossref]
- Liao D, Cai J, Barnes RW, Tyroler HA, Rautaharju P, Holme I, Heiss G. Association of cardiac autonomic function and the development of hypertension: the ARIC study. Am J Hypertens. 1996 Dec; 9(12 Pt 1):1147–56. [Pubmed] [Crossref]
- Patel PA, Diwan JS, Shah CJ, Mehta HB. Study of heart rate variability in hypertensive subjects. Natl J Integr Res Med. 2015 Jan; 6(1):1–6.
- Liao D, Barnes RW, Chambless LE, Simpson RJ Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability-the ARIC study. Atherosclerosis Risk in Communities. Am J Cardiol. 1995 Nov 1; 76(12): 906–12. [Pubmed] [Crossref]
- Bathula R, Francis DP, Hughes A, Chaturvedi N. Ethnic differences in heart rate: can these be explained by conventional cardiovascular risk factors? Clin Auton Res. 2008 Apr; 18(2):90-5. [Pubmed] [Crossref]
- Nama V, Onwude J, Manyonda IT, Antonios TF. Is capillary rarefaction an independent risk marker for cardiovascular disease in South Asians? J Hum Hypertens. 2011 Jul; 25(7):465-6. [Pubmed] [Crossref]
- Hughes AD, Bathula R, Park C, Tillin T, Wit N, McG Thom S, Chaturvedi N. Microcirculatory rarefaction in south Asians - a potential mechanism for increased cardiovascular risk and diabetes. PLoS One. 2013 Oct 7; 8(10):e76680 [Pubmed] [Crossref]

